When STREAM began, the standard of care for MDR-TB lasted up to 24 months, included an injectable agent, and had an average success rate of just over 50%. Stage 1 aimed to generate evidence to support the use of an effective new regimen that would significantly reduce treatment time for MDR-TB and reduce exposure to injectable agents, which are associated with significant side effects, including hearing loss and renal impairment. Stage 2 of the trial aims to generate evidence regarding the efficacy and safety of a shorter, all-oral regimen that could further reduce the burden of MDR-TB treatment.
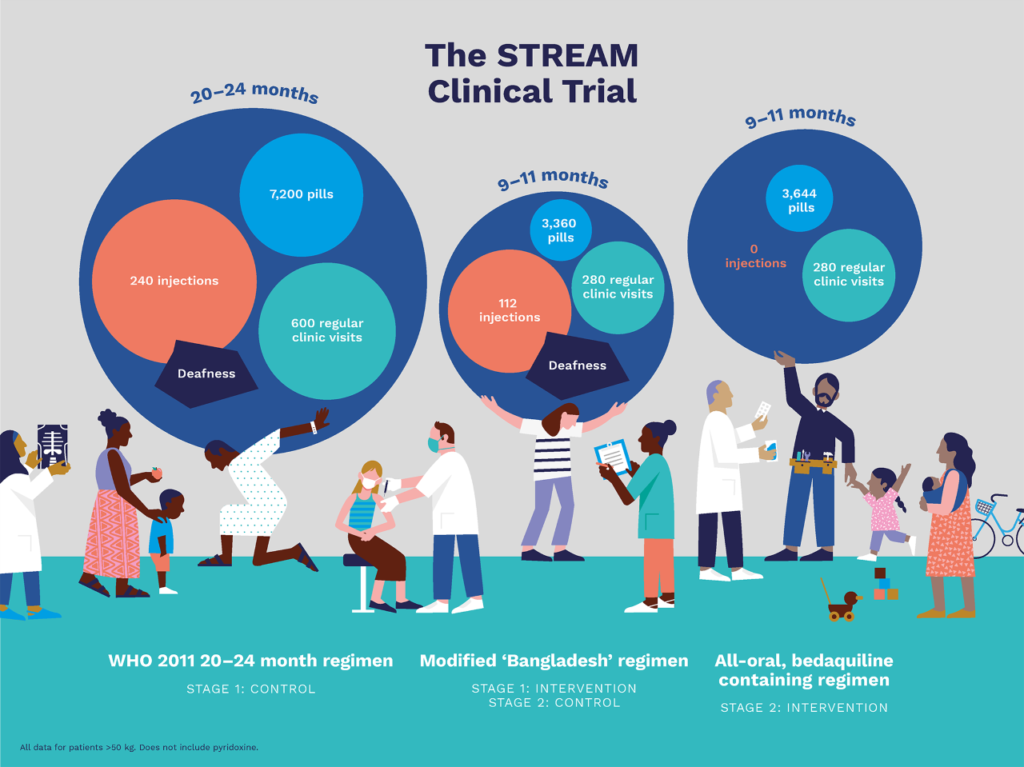
STREAM Stage 1 compared a 9–11-month MDR-TB regimen to the 20-month regimen previously recommended by the World Health Organization (WHO). The final results of Stage 1, published in the New England Journal of Medicine in early 2019, showed that the 9–11-month regimen is non-inferior to, or at least as good as, the 20-month regimen. The 9–11-month regimen reduces treatment time significantly, which also means the overall pill burden for patients decreases by approximately 60 percent.
In early 2020, the final results of the STREAM Stage 1 health economics evaluation were published online inthe Bulletin of the World Health Organization. The Stage 1 health economics evaluation demonstrated that the shorter 9–11-month regimen significantly reduced the cost of treating MDR-TB for both patients and health systems compared to the 20-month regimen. These results should be very encouraging for national TB programs as they decide whether and how to introduce shorter regimens for treatment of MDR-TB.
STREAM Stage 2 is now evaluating an all oral, bedaquiline-containing regimen that is potentially as effective and more tolerable than the injectable-containing regimens currently in use. It is also evaluating the comparative cost of the two regimens, for both the patient and the health system.
The highest quality evidence the WHO looks [for] is from randomized clinical trials … They are the number one approach for making new guideline recommendations, and results from clinical trials like STREAM help to ensure WHO recommendations … are as strong as possible.
Dr. Matteo Zignol,
Coordinator of the TB/HIV and community engagement Unit and Team Leader for the Research for TB Elimination team at the WHO Global TB Program
